To create ionic compounds, it is usually necessary to add a space between the cation and anion. This prevents FX ChemStruct trying to bond the two pieces together and clearly shows the charge of each ion.
For example:
fe so3
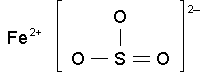
feso3
![]()
In the second example, FX ChemStruct has tried to create one molecule out of the cation and anion and become "confused". The space makes all the difference.